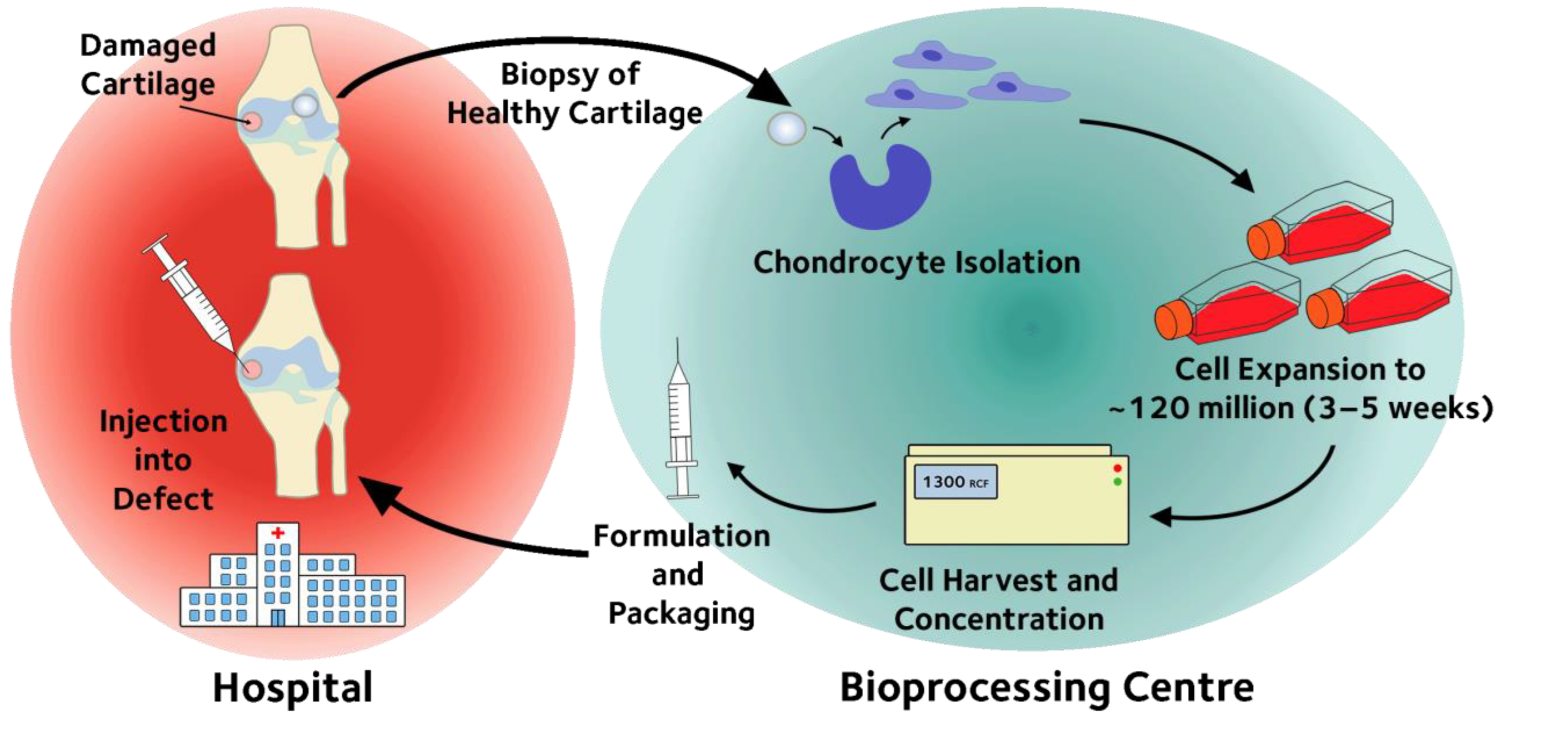
Stem-like memory T cells are generated during hollow fiber perfusion-based expansion and enriched after cryopreservation in an automated modular cell therapy manufacturing process - Cytotherapy

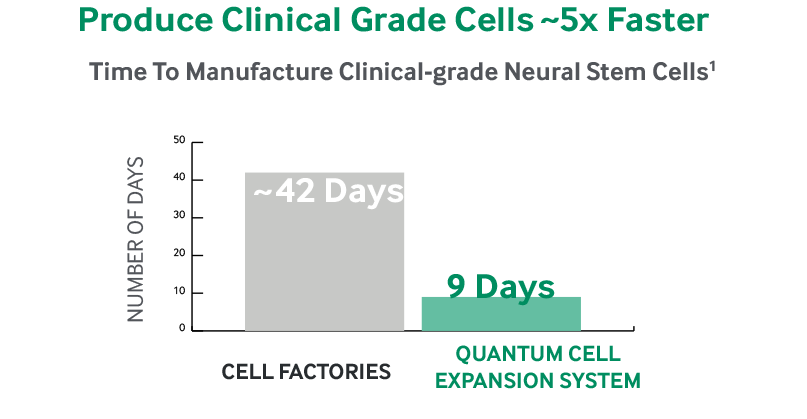
Automated Quantum Cell Expansion System (left) showing the graphic user... | Download Scientific Diagram

The WAVE bioreactor and static bags expand the same lymphocyte cultures... | Download Scientific Diagram

A Comparison of Automated Perfusion- and Manual Diffusion-Based Human Regulatory T Cell Expansion and Functionality Using a Soluble Activator Complex - Mark Jones, Brian Nankervis, Kelly Santos Roballo, Huong Pham, Jared Bushman,

Frontiers | GMP-Compliant Production of Autologous Adipose-Derived Stromal Cells in the NANT 001 Closed Automated Bioreactor

Automated Good Manufacturing Practice–compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer

Assessment of the LOVO device for final harvest of novel cell therapies: a Production Assistance for Cellular Therapies multi-center study - Cytotherapy

Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum® Cell Expansion System for the culture of human mesenchymal stem cells - ScienceDirect