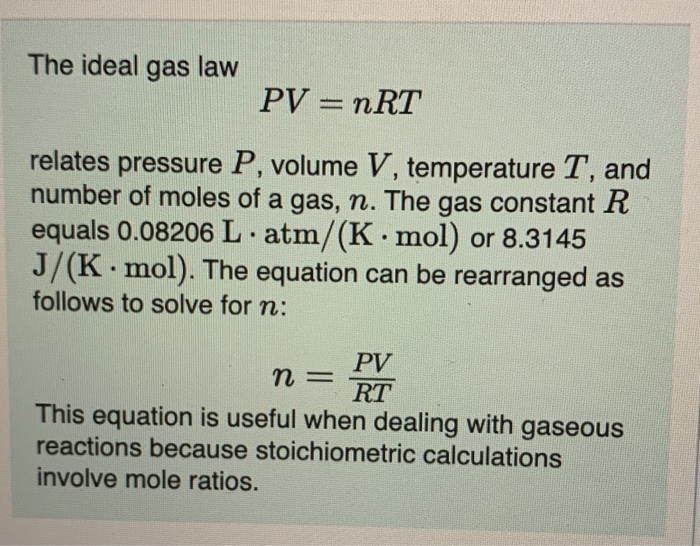
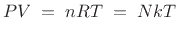Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

1 TOPIC 7: GASES Contents Properties of Gases The Simple Gas Laws The Ideal Gas Equation Gases in Chemical Reactions Mixture of Gases Kinetic-Molecular. - ppt download

The ideal gas equation. Room temperature and pressure, RTP Limitations At RTP, 1 mol of gas molecules occupies 24.0 dm 3 Conditions are not always room. - ppt download

Ideal-Gas Equation The constant of proportionality is known as R, the gas constant. © 2009, Prentice-Hall, Inc. - ppt download
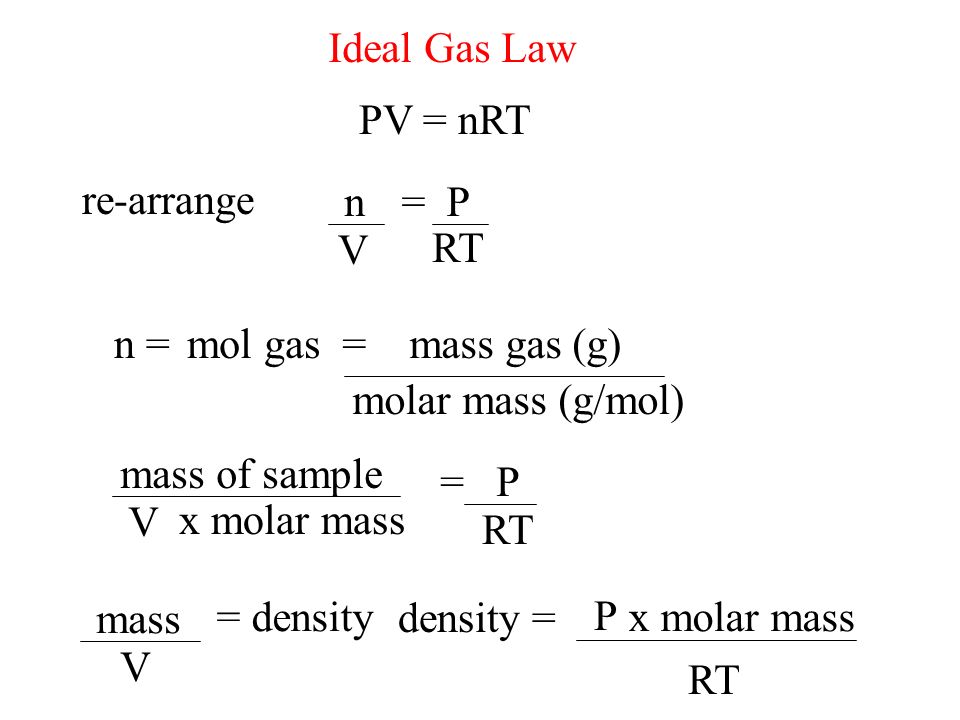
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =
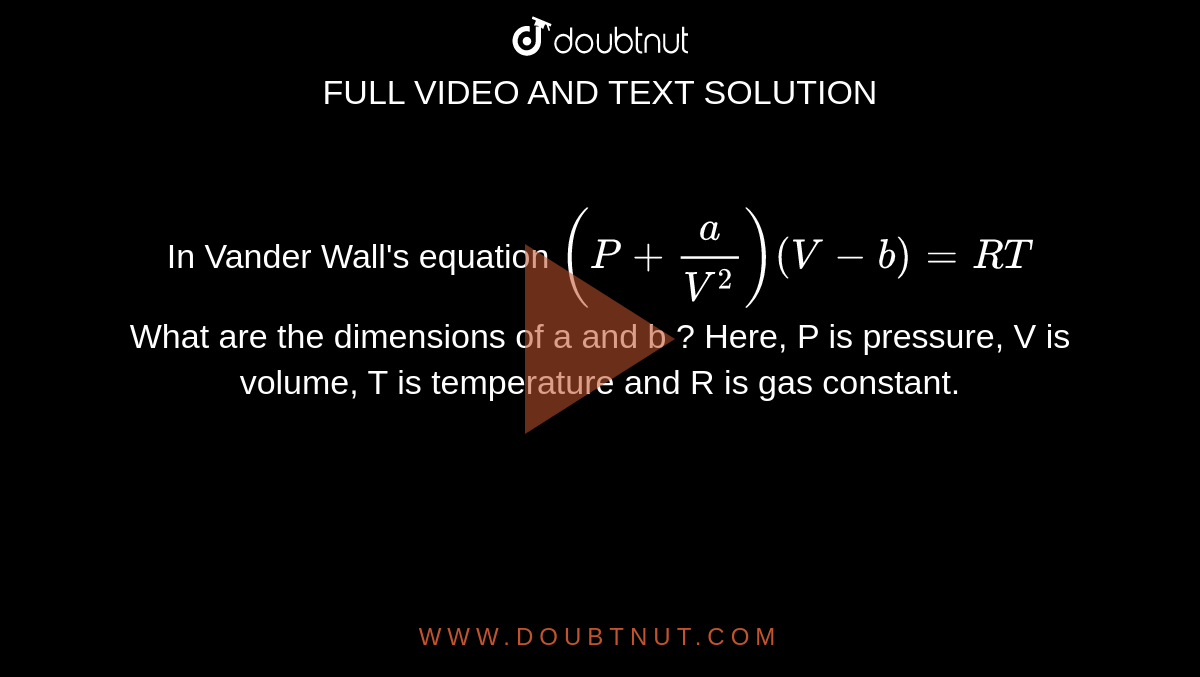
In Vander Wall's equation (P +(a)/(V^2))(V - b) = RT What are the dimensions of a and b ? Here, P is pressure, V is volume, T is temperature and R is
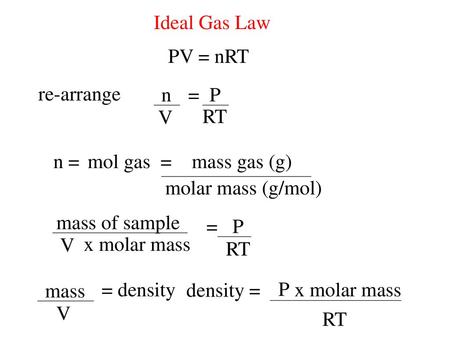
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

A gas obeys the equation of state P(V - b) = RT (The parameter b is a constant). The slope for an isochore will be:

Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =