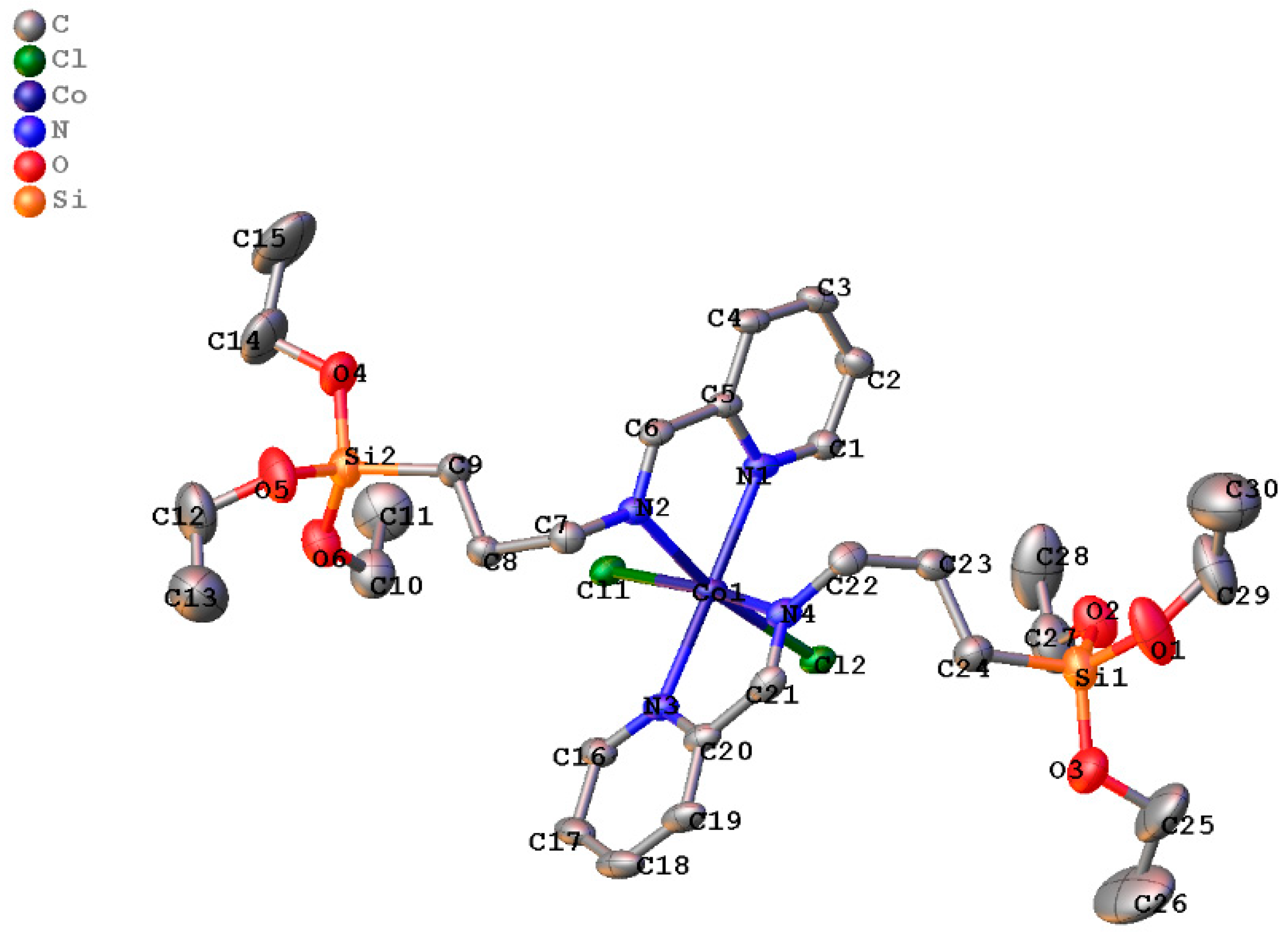
Molecules | Free Full-Text | The Transfer Hydrogenation of Cinnamaldehyde Using Homogeneous Cobalt(II) and Nickel(II) (E)-1-(Pyridin-2-yl)-N-(3-(triethoxysilyl)propyl)methanimine and the Complexes Anchored on Fe3O4 Support as Pre-Catalysts: An ...

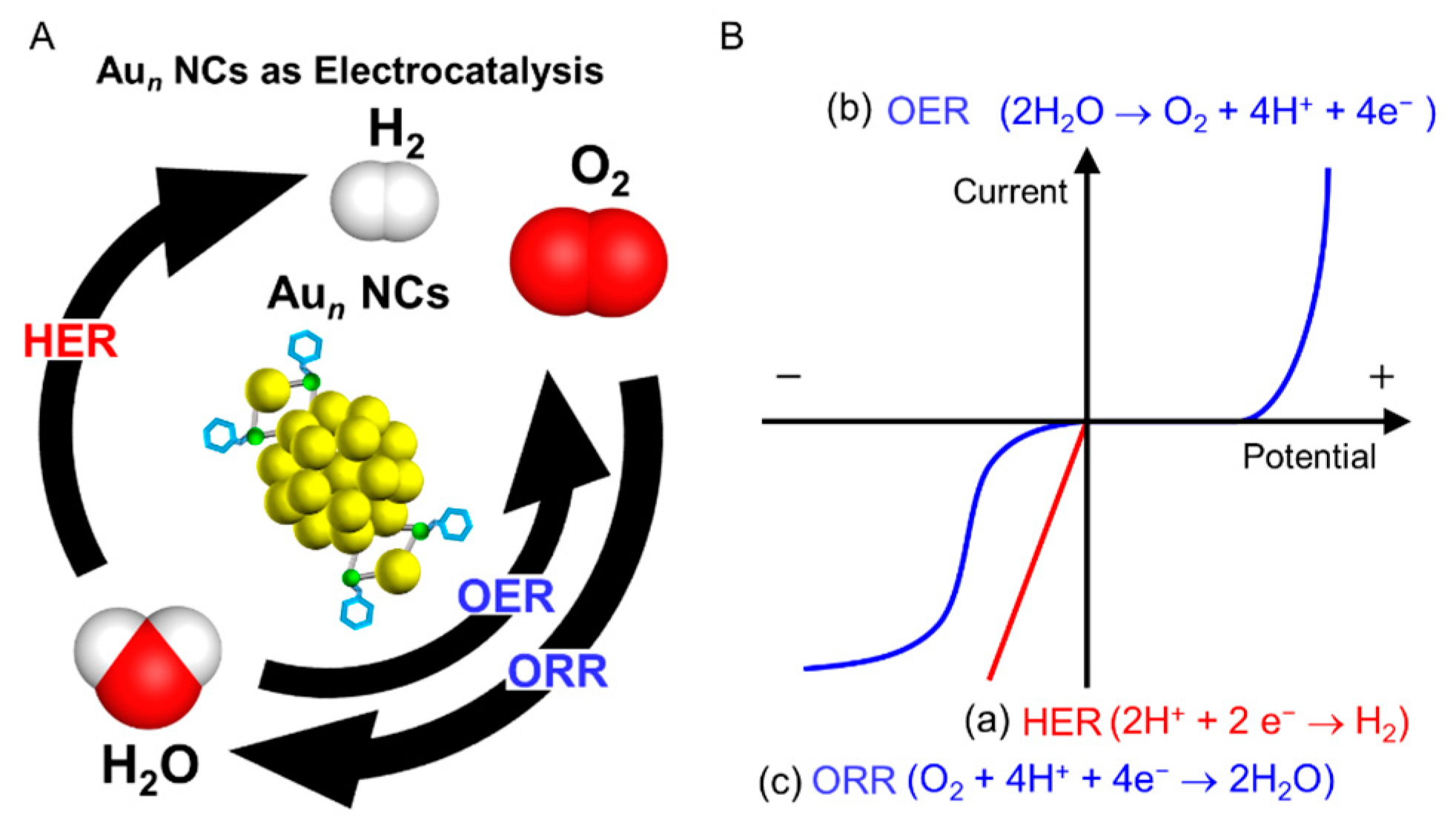
Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions | ACS Catalysis

A Distinctive Pattern for Substituent Effects on Transition Metal Centers: Enhanced Electron-Donating Capacity of Cationic Palladium Species | CCS Chem
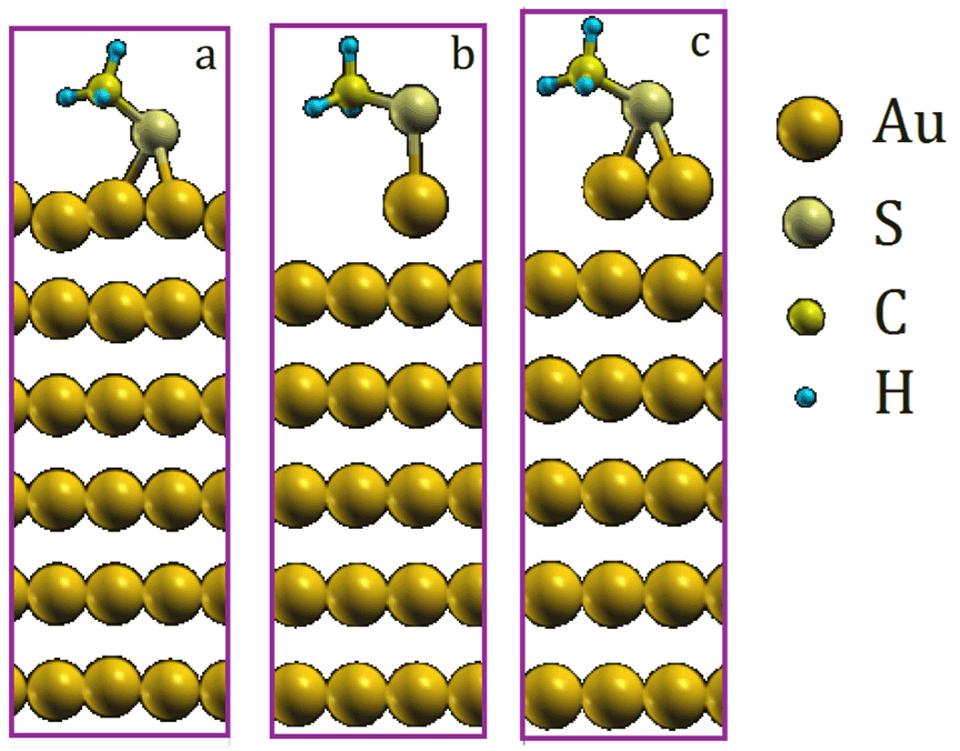
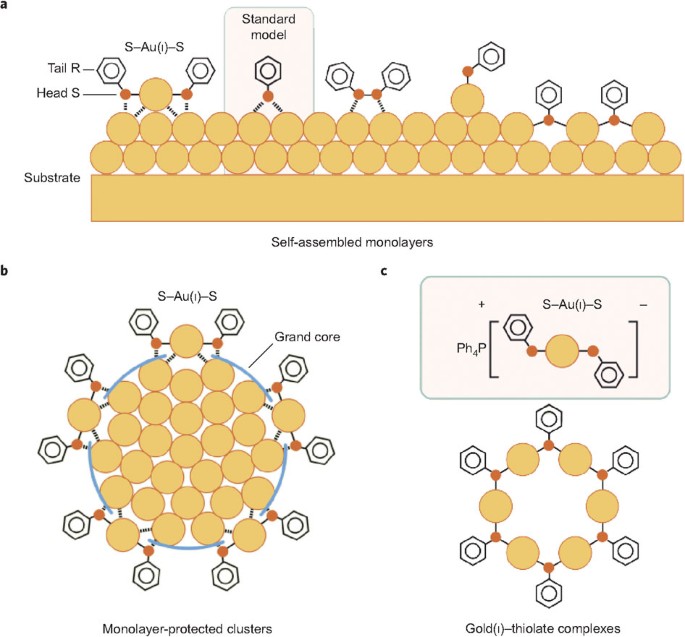
Exploring the Nature of the Au-S Bond in Thiol-Functionalized Gold – Surface Science and Technology | ETH Zurich

Cobalt–Nickel Nanoparticles Supported on Reducible Oxides as Fischer–Tropsch Catalysts | ACS Catalysis

Tuning Palladium Nickel Phosphide toward Efficient Oxygen Evolution Performance | ACS Applied Energy Materials

Incorporating Sulfur Atoms into Palladium Catalysts by Reactive Metal–Support Interaction for Selective Hydrogenation | CCS Chem

Nickel and Palladium Complexes of a PP(O)P Pincer Ligand Based upon a peri-Substituted Acenaphthyl Scaffold and a Secondary Phosphine Oxide | Inorganic Chemistry

Control of Molecular Bonding Strength on Metal Catalysts with Organic Monolayers for CO2 Reduction | Journal of the American Chemical Society
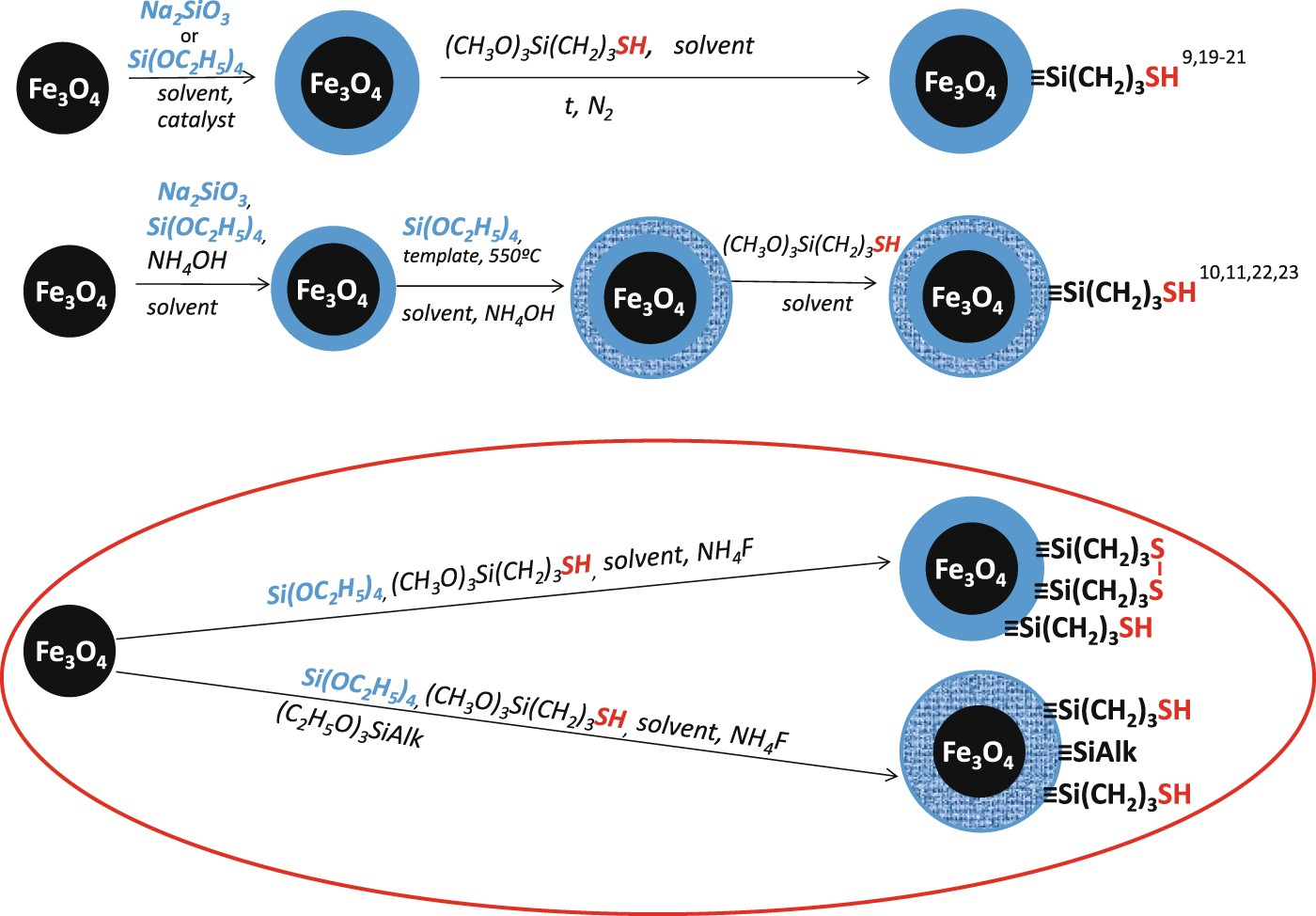
Protection of Thiol Groups on the Surface of Magnetic Adsorbents and Their Application for Wastewater Treatment | Scientific Reports

Bond Dissociation Energies of Tungsten Molecules: WC, WSi, WS, WSe, and WCl | The Journal of Physical Chemistry A

Photocatalysis in Dual Catalysis Systems for Carbon‐Nitrogen Bond Formation - Singh - 2021 - Advanced Synthesis & Catalysis - Wiley Online Library

N-Heterocyclic Carbene Complexes of Nickel, Palladium, and Iridium Derived from Nitron: Synthesis, Structures, and Catalytic Properties | Organometallics
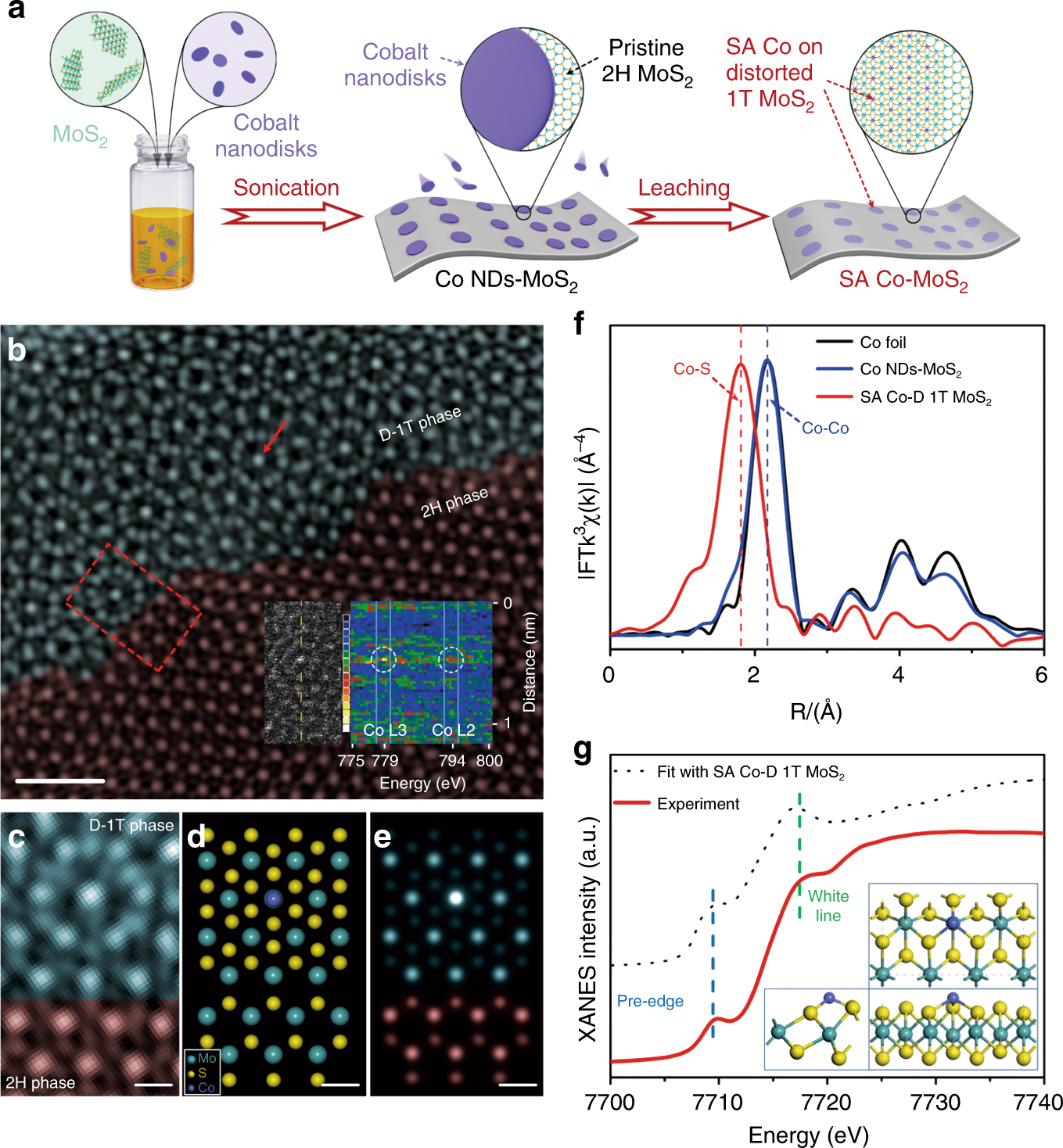
Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis | Nature Communications
Nanomaterials | Free Full-Text | Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing